Pipeline
What Is Thrombosis?
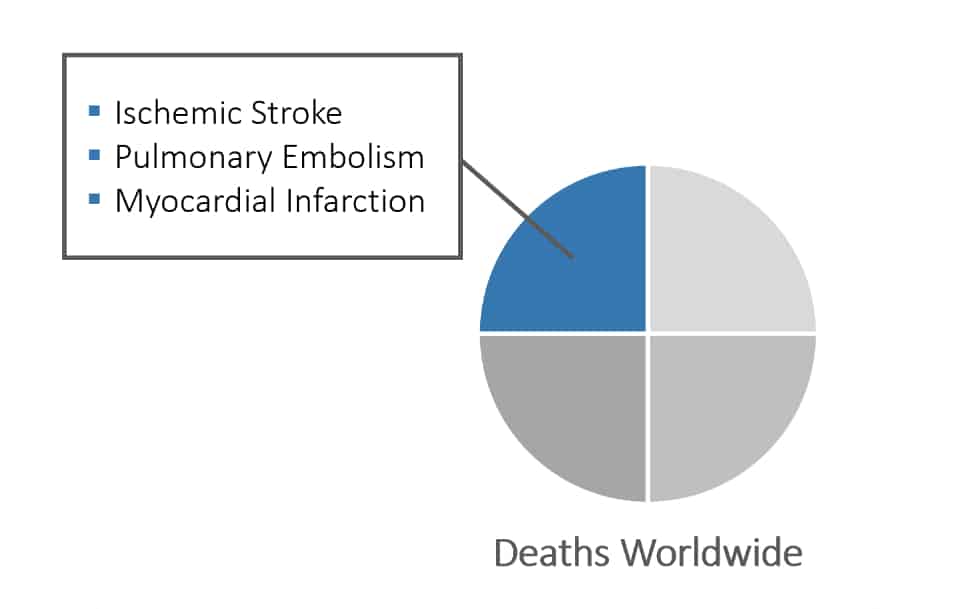
- Extremely common - the underlying cause of 1 in 4 deaths worldwide
- Leads to many conditions including ischemic stroke, pulmonary embolism and myocardial infarction
- A global lifetime risk of stroke from the age of 25 onward of approximately 25%
Acute Ischemic Stroke (AIS):
Deadly, Disabling, Costly and Undertreated
- Blood clot obstruction in a vessel in the brain
- A major cause of death and disability
- Accounts for almost 87% of all strokes
- Mortality projected to increase by 47% between 2020 and 2050
There is also evidence of increasing incidence of stroke in younger
individuals (under 55).
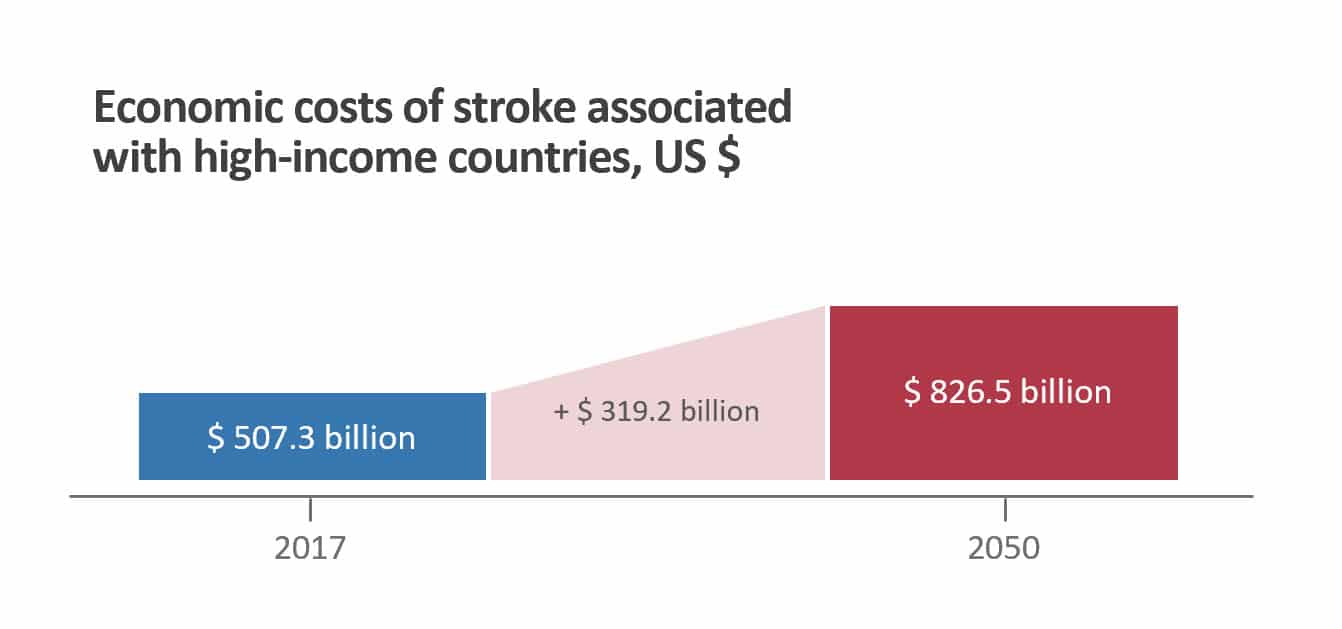
Global Economic Burden
- Stroke cost to high income countries US $ 507.3 billion in 2017
- Projected to increase to US $ 826.5 billion by 2050
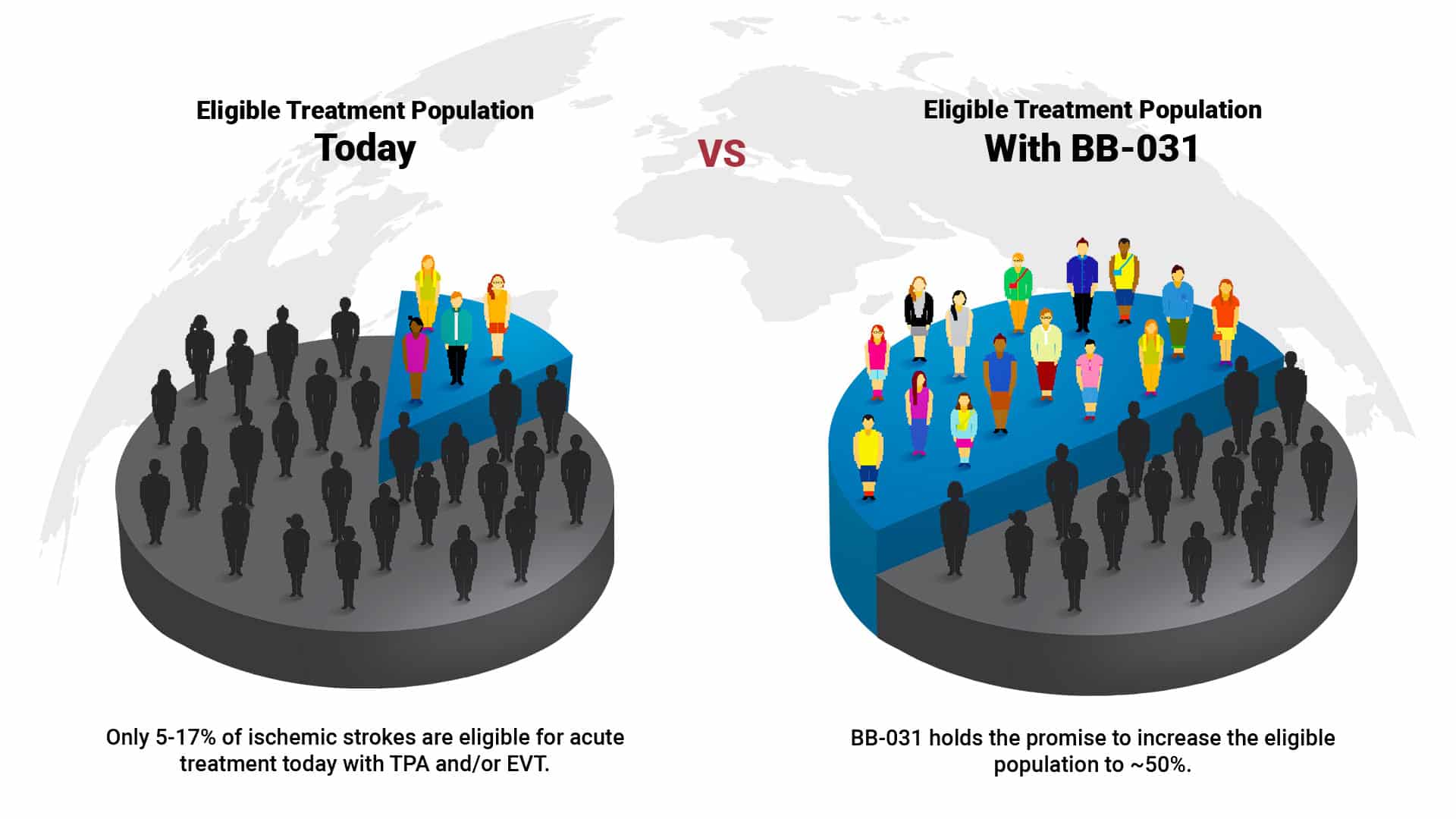
Current AIS treatments reach <15% of all patients and have significant limitations
- 80-85% of AIS patients have no effective treatment options
- Tissue plasminogen activator (TPA)
- Narrow therapeutic window, restricted to 3 to 4.5 hours (in the U.S.) post-stroke onset – a major limitation since most patients present after that time period
- High risk of intracerebral hemorrhage following treatment

- Device-based endovascular thrombectomy (EVT)
- Limited by its ability to mechanically retrieve clots found only in the large vessels of the brain
- Many acute care centers lack access to the technology due to the expense and infrastructure requirements for the surgical intervention
- Only 10% of AIS patients are treated via EVT
Our Solution
The limitations of current acute revascularization treatments in AIS mean that the vast majority of patients do not receive any acute treatment. A safe, effective, reversible drug will greatly expand access to acute revascularization treatment. Restoring blood flow quickly is the only medical intervention that is proven to improve outcomes in AIS.
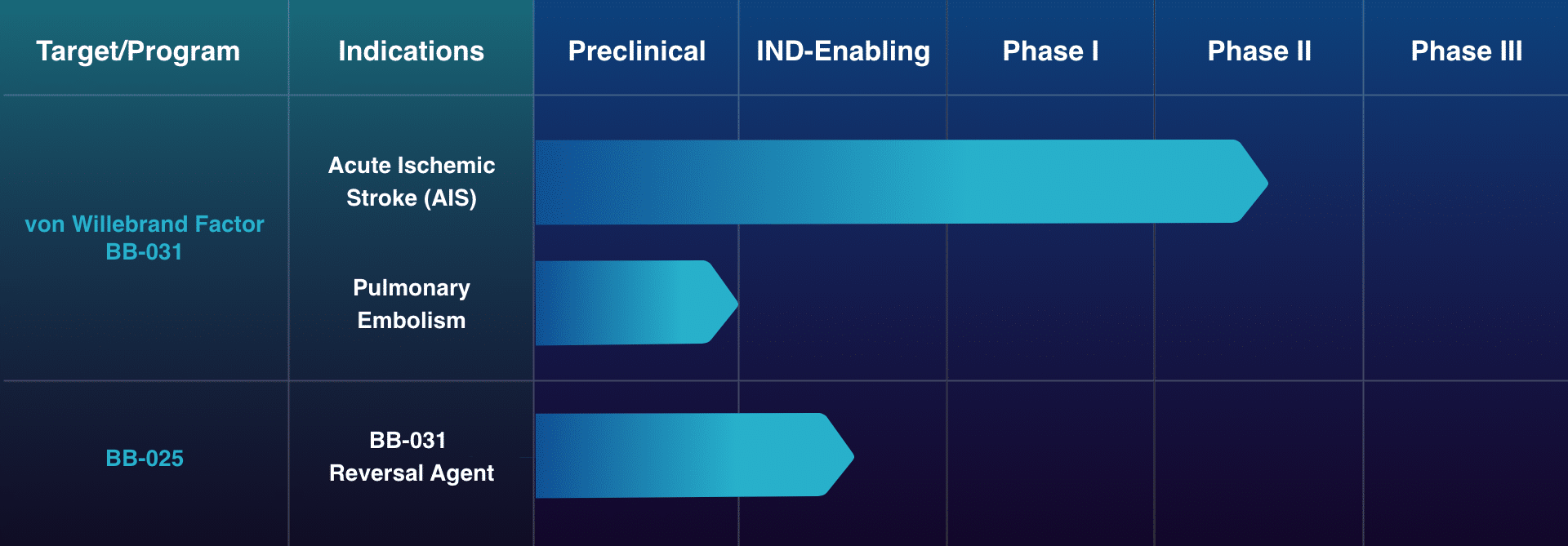
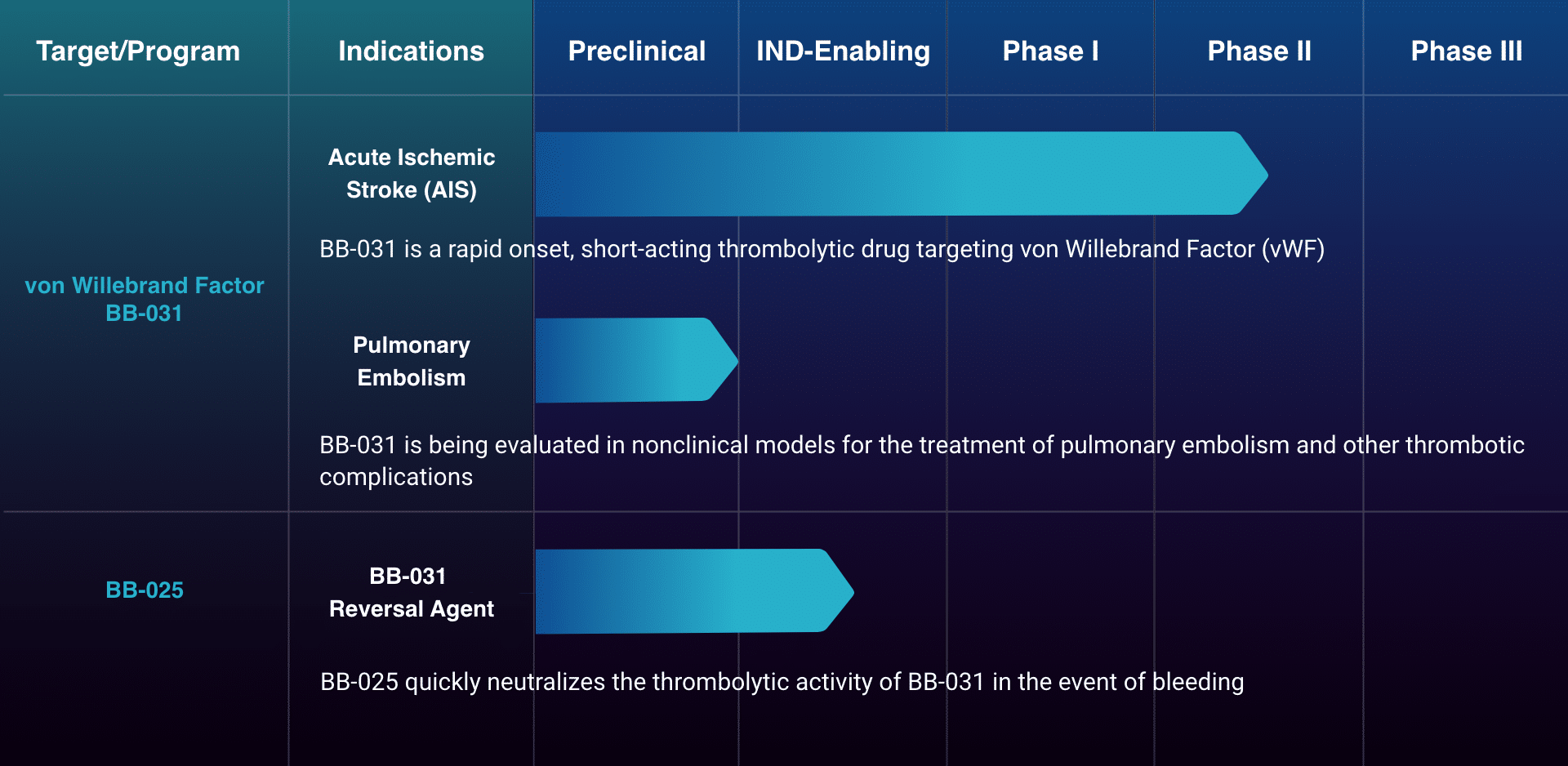
Basking’s BB-031 is a first-in-class RNA aptamer targeting von Willebrand Factor (vWF), a key driver of the blood clotting process. It is specifically designed to have a rapid-onset, be short-acting and reversible.
BB-031 is engineered to be safer and more effective, and capable of reopening blocked arteries beyond the therapeutic time window of standard of care, thus increasing the eligibility of the AIS patient population for acute revascularization.
Pipeline
The Path Forward
Enrollment of ischemic stroke patients in the Phase II RAISE trial is ongoing.
Contact us if you have an interest in participating as a trial investigator.